ISO 7886-1
Hypodermic Syringe Test
그만큼 ISO 7886-1 standard defines the performance and safety requirements for hypodermic syringes intended for single use, emphasizing aspects like leak resistance, dead space, 그리고 glide force. Manufacturers and quality assurance teams must understand the testing procedures outlined in ISO 7886-1 to ensure product compliance and patient safety.
ISO 7886-1 applies to:
-
Sterile syringes made of plastic materials.
-
Syringes used with hypodermic needles for subcutaneous or intramuscular injection.
-
Syringes intended for single use.
This standard does not apply to syringes for insulin (covered by ISO 8537), dental use (covered by ISO 7885), or syringes for infusion pumps.
ISO 7886 Testing and Its Significance
ISO 7886-1 focuses on performance requirements and test methods for hypodermic syringes, particularly those used with or without needles. These tests ensure:
1. Design and Construction
-
Transparent barrel for visual inspection of contents.
-
Graduated scale printed with permanent ink.
-
Secure plunger fit to prevent leakage or dislodgement.
2. Volume Accuracy
-
The actual delivered volume must match the nominal volume marked on the barrel within specified tolerances.
-
Accuracy is tested at 10%, 50%, and 100% of the nominal capacity.
3. Leakage Tests
-
Air leakage 그리고 liquid leakage are tested under defined pressure and volume conditions.
-
No visible leakage should occur from the barrel or between components.
4. Dead Space
-
The volume remaining in the syringe after full depression of the plunger must not exceed the specified maximum (usually ≤ 0.07 mL).
5. Plunger Function
-
The plunger should move smoothly without sticking or slipping.
-
There must be no leakage or separation under normal operating pressure.
6. Break Force and Separation Force
-
The maximum force required to break the plunger or separate the components should not fall below safety thresholds.
7. Biocompatibility
-
Materials in contact with the drug or injection site must pass ISO 10993 series biocompatibility tests.
Each test helps determine if a syringe is safe, reliable, and consistent in performance.
Hypodermic Syringe Test Methods in ISO 7886-1
1. Hypodermic Syringe Leak Test Under Vacuum (Annex B)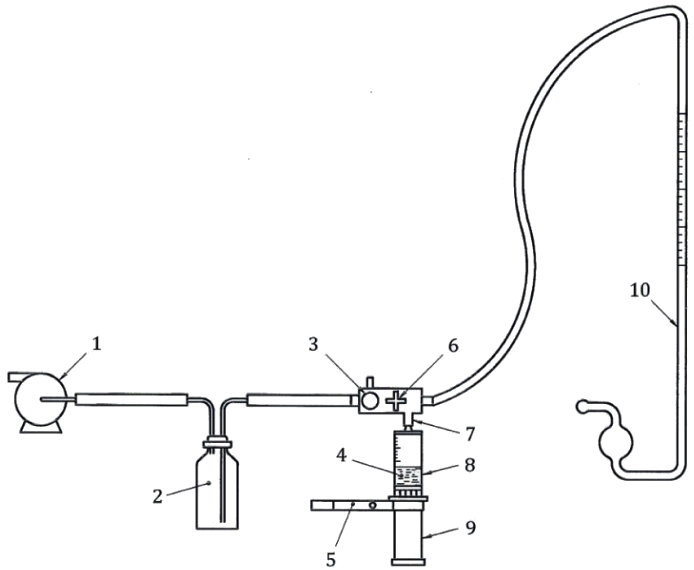
Leakage past the plunger stopper under negative pressure must be tested to avoid contamination or air intrusion. This hypodermic syringe leak test is essential for verifying airtightness.
-
Method: Fill the syringe with water (at least 25% capacity), clamp the plunger, and apply -88 kPa vacuum.
-
Observation: Inspect for any air leaks or detachment of the plunger stopper.
Recommended Equipment:
셀 인스트루먼트' SLT-02 Syringe Leak Tester offers precise vacuum control and automated readings, ensuring compliance with Annex B.
2. Hypodermic Syringe Liquid Leak Test Under Compression (Annex D)
그만큼 hypodermic syringe liquid leak test examines syringe integrity under axial and lateral mechanical stress.
-
Method: After sealing the nozzle, a side force is applied to deflect the plunger, followed by axial compression to simulate user operation.
-
Observation: Monitor for fluid leakage at pressures of 200 kPa and 300 kPa.
Recommended Equipment:
사용 SPPT-01 Syringe Positive Pressure Tightness Tester from Cell Instruments to apply controlled mechanical forces and monitor for any failure in seal integrity.
3. Syringe Dead Space Test: Minimizing Medication Waste (Annex C)
Dead space refers to the volume of fluid that remains in the syringe after complete expulsion. High dead space can lead to drug wastage and inaccurate dosing.
-
Method: Weigh the empty syringe, then weigh it after being filled and emptied of water.
-
Calculation: The difference in weight indicates dead space in mL.
4. Glide Force Test for Syringe Piston (Annex E)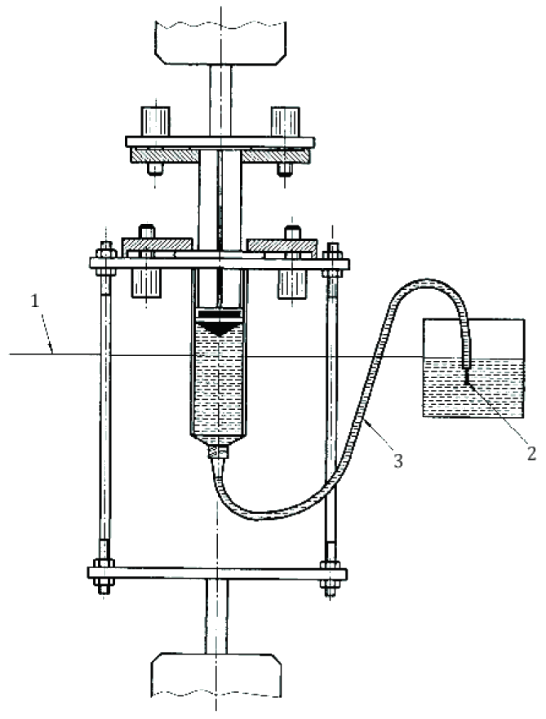
Smooth piston movement is critical for user comfort and precise dosage control. The 활공력 테스트 measures the force required to operate the piston consistently across the full stroke.
-
Method: A mechanical testing machine depresses the plunger at a fixed rate, recording real-time resistance.
-
Purpose: Verifies the syringe usability and consistency across lots.
Recommended Equipment:
Cell Instruments MST-01 의료용 주사기 테스터 is ideal for 활공력 테스트 applications. It records force profiles and complies with ISO 7886-1 accuracy requirements.
Why Choose Cell Instruments for ISO 7886 Testing?
Cell Instruments offers a complete suite of syringe testing solutions to help manufacturers meet and exceed ISO 7886-1 requirements. Our equipment is designed for:
-
High precision and automation
-
Compliance with international standards
-
Easy operation and data recording
-
Flexible customization to meet specific test setups
Whether you’re validating product design or managing batch release testing, SLT-01, SPPT-01, 그리고 MST-01 deliver reliable and repeatable results tailored to the medical device industry’s needs.
Achieving compliance with ISO 7886-1 is not just a regulatory hurdle—it’s a commitment to quality and patient safety. By understanding and implementing essential tests like the hypodermic syringe test, 누출 테스트, syringe dead space test, 그리고 활공력 테스트, manufacturers can confidently release products to the market. Equip your lab with Cell Instruments’ advanced testers to streamline testing and ensure accuracy across all stages of syringe production.
For technical consultations, instrument selection, or customized solutions, contact Cell Instruments today.

